Pipeline – LARS symptoms (rectal installation)
RepoCeuticals has developed a medicinal product (rectal solution) for the relief of the symptoms and the chronic consequences of low anterior resection syndrome – LARS.
RepoCeuticals rectal solution for LARS is expected to have a tangible beneficial impact on the principal symptoms of LARS, moderating hyperactive bowel movements, lessening pain and enhancing sleep.
RepoCeuticals has conducted a clinical trial in collaboration with Danish University Hospitals to assess its efficacy in relieving the symptoms and chronic consequences of LARS.
Data is being analyzed.
Indications
- Relief of the symptoms and the chronic consequences of Low Anterior Resection Syndrome.
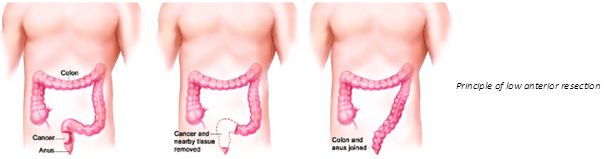
There are over 100,000 new cases of LARS per year in the EU and the USA combined. More than 1 million people suffers from the symptoms of LARS.
LARS produces a number of unpleasant bowel symptoms, which affect over 85% of all patients who have a proctectomy because of rectal cancer. When the rectum is removed, the colon is connected directly either to the anus or the rectal stump, in an attempt to avoid colostomy and restore more or less normal bowel function. Unfortunately, the results are disappointing, and most patients go on to suffer something resembling an extreme irritable bowel syndrome, with frequent flatus and thin stools, incontinence or fecal urgency, alternating with obstipation and a feeling of incomplete evacuation, all accompanied by stomach pains, sleep problems and chronic fatigue. LARS is extremely damaging to the quality of life of the affected patients, and there is unfortunately no effective relief available today.

