Pipeline – Radiation proctitis
RepoCeuticals’ medicinal product (rectal solution) for the prevention and treatment of radiation damage to pelvic organs – rectum.
RepoCeuticals’ rectal solution is one of a series of products expected to exert a relevant therapeutic effect to prevent and treat radiation damage to pelvic organs as a result of radiotherapy to the pelvic region, in this case by local application to the rectum.
Common to the radiation protection projects is also that many cytotoxic drugs used to treat cancers may cause organ damage similar in symptoms and pathology to radiation injury, and the use of both types of cancer treatment in the same patient greatly increases the chances and severity of such organ damage.
Indications
- Pelvic external beam radiotherapy for conditions such as carcinomas of the prostate gland, rectum or anus, or other pelvic organs such as the uterine cervix and endometrium.
- Pelvic brachytherapy (internal implant radiotherapy) for these conditions.
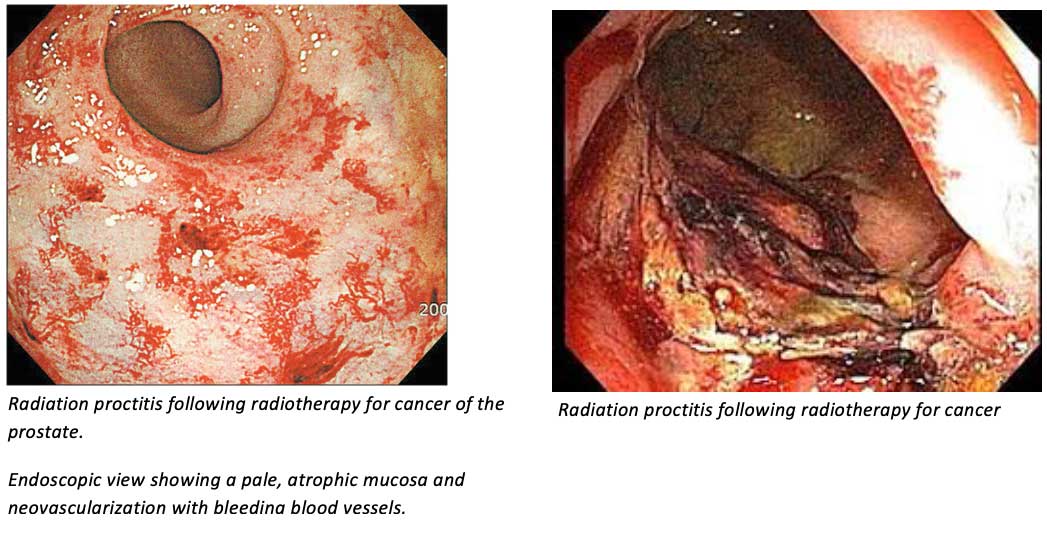
There are over 200,000 cases of pelvic radiotherapy per year in EU and USA; these are all candidates for prophylactic treatment with RepoCeuticals’ topical melatonin preparations to prevent radiation damage to pelvic organs.
Radiation proctitis (inflammatory damage to the rectal mucosa) affects approximately 75% of these patients. The Macmillan Cancer Support charity estimates that 90,000 patients in the UK are currently affected by radiation proctitis.
On average, the patients receiving radiotherapy are exposed to 10-20 doses of radiation. Patients exposed to pelvic radiotherapy may need protective treatment to the rectum and bladder, as well as to the vagina in women, leading to 180,000 – 540,000 treated patient per year. Each patient will need from 14 to 20 treatments giving a total of 2.5 – 10.8 million doses per year.

