Pipeline – Radiation cystitis
RepoCeuticals has developed a medicinal product (solution for intravesical instillation) for the prevention and treatment of radiation damage to pelvic organs – bladder.
RepoCeuticals’ solution for intravesical instillation is expected to exert a relevant therapeutic effect to prevent and treat radiation damage to a pelvic organ as a result of radiotherapy to the pelvic region, in this case by local application to the urinary bladder.
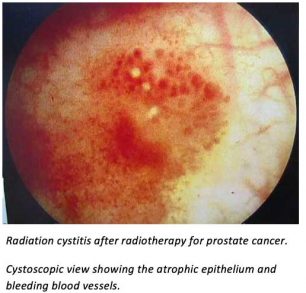
The general principles and purpose of the treatment are the same as for the rectum and vagina. The pathology and consequences of radiation damage to the urinary bladder have been described above in the section on “Radiation damage due to radiotherapy to the chest and pelvic regions”. Radiation cystitis is particularly frequent in men treated for prostatic cancer by pelvic radiotherapy, but the preventive use of the protective preparation means that it should be applied to all patients receiving pelvic radiotherapy.
Indications
- Pelvic external beam radiotherapy for conditions such as prostatic carcinoma, carcinoma of the bladder, carcinoma of the rectum or anus, as well as cervical and endometrial carcinoma of the uterus.
- Pelvic brachytherapy for these conditions.
- Cystitis caused or exacerbated by cytotoxic cancer chemotherapy.
There are over 200,000 cases of pelvic radiotherapy per year in EU and USA; these are all candidates for prophylactic treatment with RepoCeuticals’ topical melatonin preparations to prevent radiation damage to pelvic organs.
Radiation cystitis frequencies may vary from 2% to 65% depending on the degree of severity and site of the cancer. Mean figures for high-grade radiation cystitis may be 14% for cancer of the prostate and 18% for bladder cancer.
On average, the patients receiving radiotherapy are exposed to 10-20 doses of radiation. Patients exposed to pelvic radiotherapy may need protective treatment to the rectum and bladder, as well as to the vagina in women, leading to 180,000 – 540,000 treated patient per year. Each patient will need from 14 to 20 treatments giving a total of 2.5 – 10.8 million doses per year.

